WASHINGTON, Aug 11 (NNN-AGENCIES) — New data published in the Morbidity and Mortality Weekly Report and gathered by the Centers for Disease Control and Prevention (CDC) showed that 99 percent of monkeypox cases in the United States are in males.
The report showed that 94 percent of those male infected patients reported male-to-male sexual or close intimate contact during the three weeks before symptom onset, and racial and ethnic minority groups appear to be disproportionately affected.
Moreover, the data from the report, which was gathered from reported cases of the virus in the United States from May 17 to July 22, showed that clinical presentations of some patients differed from typical monkeypox, with fewer persons experiencing prodrome and more experiencing genital rashes.
These epidemiologic details should guide treatment and vaccine protocols, the authors of the study said, adding “current findings indicate that community transmission of monkeypox is widespread and is disproportionately affecting gay, bisexual, and other men who have sex with men; this is consistent with data reported from other countries.”
It noted that although the largest proportion of cases have occurred in white persons, Black and Hispanic persons, who represent 34 percent of the general population, accounted for more than one half (54 percent) of confirmed monkeypox cases.
As of Monday, the United States had the world’s most number of confirmed cases of monkeypox, which stood at 8,934. It is likely that the figure is a severe undercount because of the lack of testing.
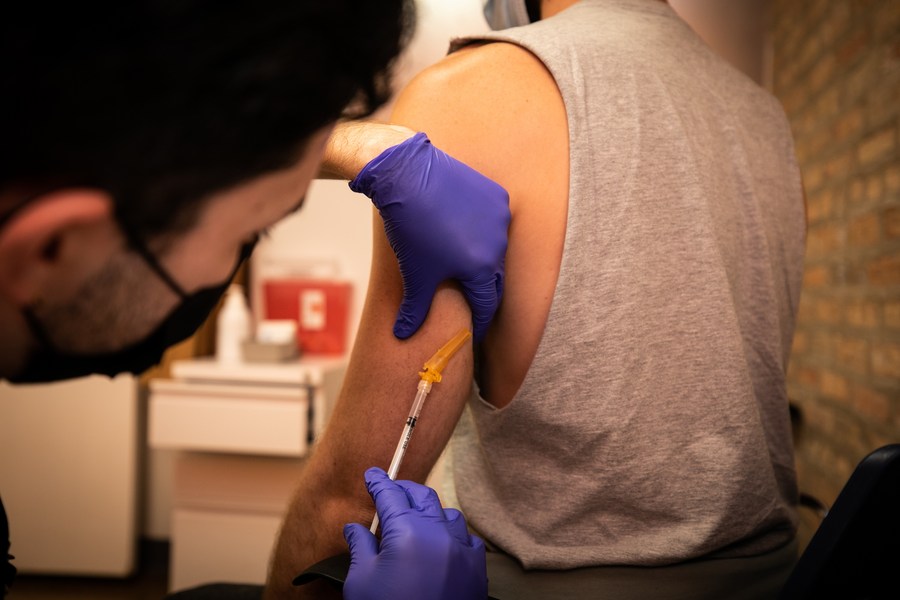
Meanwhile, US health authorities authorized a new procedure for injecting the monkeypox vaccine that should make it possible to inoculate more people with the same amount of the drug, at a time when doses are running short in the country.
The Food and Drug Administration also authorized giving the vaccine to people under the age of 18 who are considered to be at high risk of infection.
For those over 18, health workers will now be able to administer the vaccine differently, via an intradermal injection — that is, between the upper layers of the skin — and not with a deeper, subcutaneous injection.
The new method will “increase the total number of doses available for use by up to five-fold,” the FDA said in a statement.
Two injections, four weeks apart, will still be necessary.
The FDA said it was drawing on data from a 2015 clinical trial that showed a similar immune response in people given a subcutaneous injection compared to those given a fifth of the dose via an intradermal shot.
At present, some 620,000 doses of the vaccine — manufactured by Bavarian Nordic, and marketed under the name Jynneos in the United States — have been distributed across the country.
Another 440,000 additional doses are still to be distributed, which could allow up to 2.2 million injections under the new strategy.
The government has also ordered an additional five million doses, which will start arriving from September and run through 2023, affording the potential to administer 25 million doses.
The decisions came after the FDA issued an emergency use authorization for the vaccine, a move that itself followed the declaration of a public health emergency last week.
For the authorization in minors, the FDA said it had reviewed safety data for the vaccine, as well as data for another vaccine given in children against smallpox.
“We feel very comfortable with the safety of the approach,” said Peter Marks of the FDA at a press conference, noting a recent increase in the number of children who have potentially been exposed to infected people.
The United States has registered nearly 9,000 cases of monkeypox, a fifth of them in New York state. The vast majority of cases involve men who have had sex with men. — NNN-AGENCIES





